AirMini™
Portable CPAP machine
Small, smart CPAP device for effective1-4 therapy at home or on the move. AirMini features HumidX waterless humidification*, ActiveAir technology and powerful AutoSet and AutoSet for Her algorithms. An intuitive app provides easy set up and ongoing support.





Small
Travel easily with your therapy. AirMini is the smallest CPAP device ever manufactured.**
Smart
Stay connected. The AirMini app allows you to customise your therapy comfort to your individual needs and view your progress with ease.
Proven1-4
Rest assured, AirMini features the same established algorithms as ResMed’s AirSense range of sleep therapy devices, including the AutoSet and AutoSet for Her.
Effective waterless humidification

Enjoy the warmth and comfort of humidification without hassle.*
Our all-new HumidX system delivers a similar5 level of humidification to our market-leading bedside devices with the added convenience of being compact and waterless.
Masks designed for you

AirMini has been designed to work exclusively with our latest range of mask products. These include the AirFit F30, N20 and F20 series of masks, as well as the P10 for AirMini and AirFit N30 for AirMini.
Choose from a range of masks designed to deliver quiet, comfortable and stable therapy performance with your AirMini device.
Stay connected

The AirMini smartphone app enables you to manage your comfort settings and view your therapy progress. It also helps ensure you are swiftly set up for therapy, with guided setup videos and daily sleep scores always just a menu screen away.
Accessories for your lifestyle
With a variety of optional accessories, the AirMini system is designed to suit your lifestyle.

HumidX system
Exclusive to AirMini, HumidX , HumidX Plus and HumidX F20 are small heat and moisture exchangers (HME) that are designed to provide comfortable and effective humidification.
AirMini mount system
The ResMed mount system allows the AirMini device to be secured to various points such as a bedframe, bedside table, wall or aeroplane seat pocket.

AirMini travel bag
Take your sleep therapy everywhere you go with the AirMini travel bag. The whole solution fits inside this small, compact bag, so your AirMini is always ready when you are.
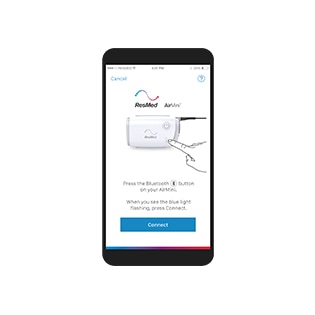
How do I set up my AirMini device?
How do I maintain and clean my AirMini system?
How do I add optional humidification to my AirMini machine?
How do I set up my AirMini machine?
- The first time you pair your machine with your smart device, you will need to authenticate it by entering the four-digit key located on the bottom of your machine. Alternatively, you can use the camera on your smart device to capture the QR code located on the bottom of the machine.
- The Bluetooth connection icon will appear in the top right corner of the screen when they are successfully connected.
- Enter the Sleep screen by pressing Sleep on the Navigation tab.
- The Sleep screen is split into two tabs: Therapy and Mask Fit. You can toggle between the Therapy screen to access comfort settings, and the Mask Fit screen to check for air leaks around your mask.
- To start therapy, press start on your Therapy screen.
- To stop therapy, press stop from the same menu.
- Enter the Sleep screen and click Options in the Therapy screen.
- Tap the setting you wish to adjust.
- Use the spinner or switches to select the required setting.
- Perfom this process for as many settings as needed.
- Enter your Dashboard screen by pressing Dashboard in the Navigation tab. If your AirMini machine is connected to the AirMini app, the Dashboard will provide summary data and a sleep score for your most recent therapy session.
- You can also access sleep score information for previous days by clicking the dropdown arrow next to the current date.
- Tap on any therapy session to view your sleep score and sleep data for that date.
FAQ
The AirMini is designed to support simplicity and a rich user experience. To achieve this, we designed AirMini with one-button operation for simplicity and an accompanying app that unlocks an engaging, customisable therapy experience.
If your smart device disconnects for any reason, the AirMini will continue to deliver therapy uninterrupted. The data will be transmitted to your smart device when it next connects to the AirMini.
Your smart device should automatically reconnect to the AirMini machine every time it is within range, as long as you have successfully paired your devices once and Bluetooth® is activated and visible on your smart device. If necessary, you can also connect by simply tapping the Bluetooth® icon on the top right corner of the app’s home screen. A prompt to connect your smart device with the AirMini machine will appear. The Bluetooth® icon will be visible in the app and the Bluetooth® LED on the machine will turn blue and remain blue when it’s paired with your smart device.
Your sleep score and sleep statistics can be viewed in the AirMini App.
ActiveAir and HumidX are new technologies that have been developed especially for AirMini. The 20 series masks, AirFit N30 for AirMini and AirFit P10 for AirMini have been designed to meet the specific requirements of these technologies and are the only masks that are currently compatible with the AirMini system.
No. HumidX components cannot be cleaned and should be replaced 30 days after you open the packaging.
ResMed does not recommend using HumidX components if the packaging was opened more than 30 days ago. If HumidX is used more than 30 days after opening, there may be a deterioration in cleanliness, humidification performance and breathing comfort that could affect the safe and efficacious use of AirMini. Please ensure your HumidX and other components of the AirMini system are regularly maintained as per the user instructions.
Yes, the design of the AirMini machine accepts an AC input range of 100-240V and a DC output of 24V. However, to ensure your AirMini machine performs properly, we recommend purchasing the correct AirMini power supply unit for the country you are visiting. Please contact your care provider for more information.
AirMini’s optimal performance temperature range is 5°C to 35°C (41°F to 95°F). The operating temperature range is similar to that of the AirSense 10 machine. Optimal performance cannot be guaranteed if the AirMini is used outside the recommended temperature range.
The AirMini app shows you information about your therapy status and progress. The sleep screen displays current pressure in real-time. The sleep dashboard shows your sleep report and therapy progress. Your sleep data and therapy score can be accessed from the dashboard screen once therapy is complete.
Shop online
Need new equipment, accessories or spare parts? It’s quick and convenient to shop on the ResMed online store from anywhere, at any time. Register for our membership programme and you’ll also receive information on our latest products and promotions.
References:
* HumidX and HumidX Plus are only compatible with AirFit N20, AirTouch N20, AirFit N30 and P10 for AirMini. HumidX F20 is only compatible with AirFit F20 and AirTouch F20.
** As of the 6th February 2020. 136 mm (W) x 84 mm (D) x 52 mm (H), 250 cm³.
- Netzel et al. APAP device technology and correlation with patient compliance. Somnologie – Schlafforschung und Schlafmedizin 2014; DOI: 10.1007/s11818-014-0662-0.
- Isetta et al. Comparative assessment of several automatic CPAP devices responses: a bench test study. ERJ Open Res 2015;1:00031-2015.
- Zhu et al. All APAPs are not equivalent for the treatment of sleep disordered breathing: a bench evaluation of eleven commercially available devices. J Clin Sleep Med 2015 11(7):725-34.
- Isetta V et al. Novel Approach to Simulate Sleep Apnea Patients for Evaluating Positive Pressure Therapy Devices. PLoS One. 2016;11(3):e0151530. Published 2016 Mar 15.
- Alison Hansford et al. Performance of a Mask System Incorporating a Heat and Moisture Exchanger. 2017 ResMed Science Center, white paper.